Bioequivalence: What It Means for Generic Drugs and Your Health
When you pick up a generic pill, you’re not just saving money—you’re getting a drug that’s been proven to do the same job as the brand-name version. That’s thanks to bioequivalence, the scientific standard that proves two drug products release the same amount of active ingredient into your bloodstream at the same rate. Also known as therapeutic equivalence, it’s the reason your pharmacist can swap out a $200 brand drug for a $10 generic without your doctor needing to rewrite the prescription. This isn’t guesswork. It’s a strict, FDA-backed process that compares how your body absorbs and uses the drug—measured by blood levels over time—to make sure there’s no meaningful difference in how it works.
Behind every generic drug you take lies a battery of tests. Companies don’t just claim their version is the same—they have to prove it. They give the drug to healthy volunteers, track blood concentrations for hours, and compare the results against the original brand. If the generic’s absorption rate and peak levels fall within 80% to 125% of the brand’s, it’s approved. That tiny window ensures you won’t get too little (which could make the drug ineffective) or too much (which could cause side effects). This matters most for drugs with narrow therapeutic windows—like blood thinners, seizure meds, or thyroid pills—where even small changes can throw your system off balance. And it’s why you’ll see posts here about generic drug entry, how legal challenges under the Hatch-Waxman Act let cheaper versions reach the market faster, and why drug absorption, the core of bioequivalence testing isn’t just a lab detail—it’s your safety net.
But bioequivalence doesn’t mean every generic is identical in every way. Fillers, coatings, and manufacturing processes can differ, and while they don’t affect how the drug works, they can sometimes cause minor side effects in sensitive people. That’s why some patients notice a change when switching brands—even if the active ingredient is the same. The good news? Most people never feel a difference. And when they do, it’s usually something small, like a change in pill size or a different taste. What you’ll find in the posts below are real-world stories and science-backed breakdowns on how bioequivalence affects everything from antibiotics to cholesterol meds, how patent battles shape your access to generics, and what to do if you think a switch isn’t working right. You’ll learn how to spot when a generic might need extra attention, why some drugs are harder to copy than others, and how regulators keep the system honest. This isn’t just about cost—it’s about trust. And the data is clear: when bioequivalence is done right, you get the same results, for a fraction of the price.
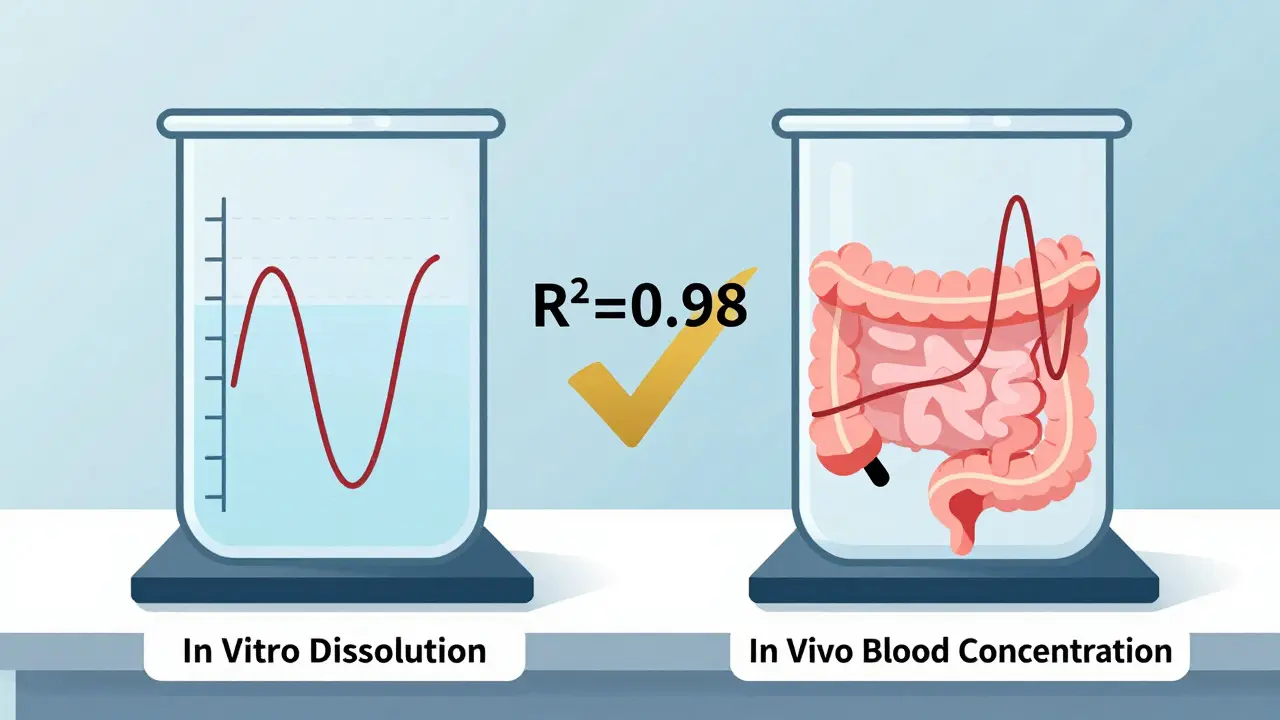
IVIVC and Waivers: How In Vitro Methods Are Replacing In Vivo Testing for Bioequivalence
- Feb, 7 2026
- 8
IVIVC uses lab-based dissolution tests to predict how drugs behave in the body, replacing costly human bioequivalence trials. Learn how it works, where it's used, and why regulators are pushing for wider adoption.
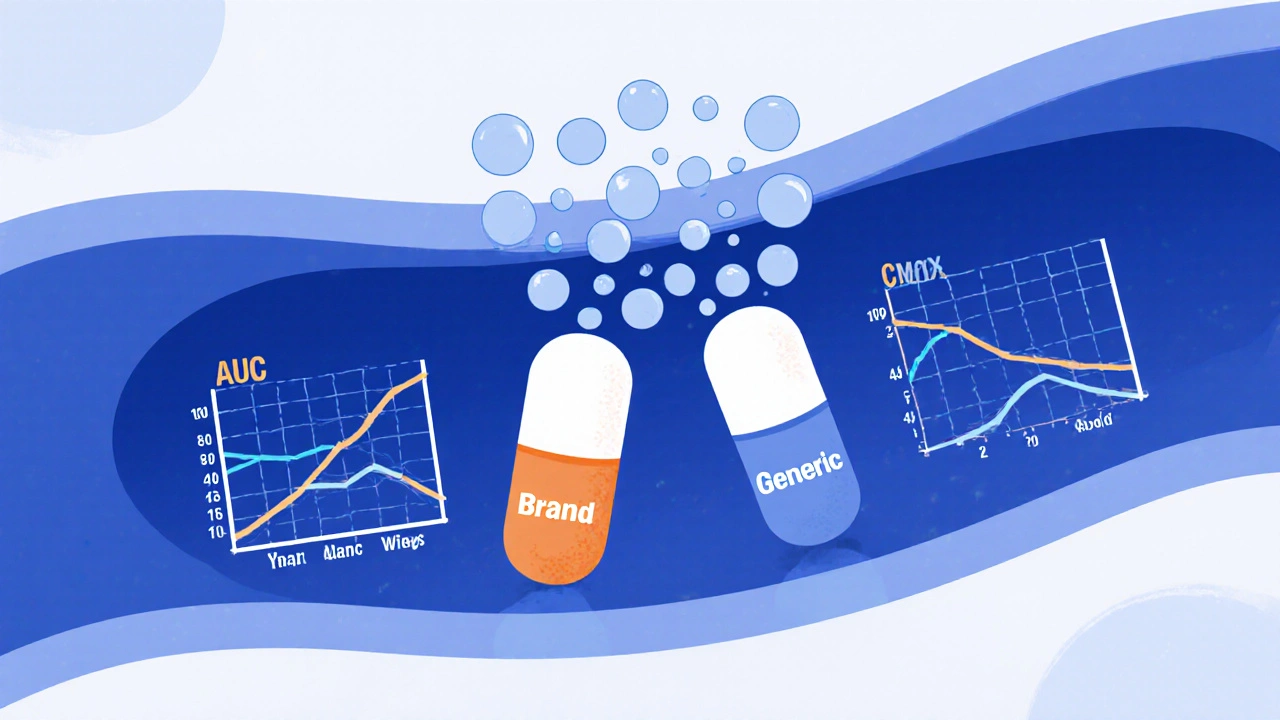
How the FDA Ensures Generic Drugs Work the Same as Brand-Name Medications
- Nov, 26 2025
- 8
The FDA ensures generic drugs work the same as brand-name medications through strict bioequivalence testing, identical active ingredients, and rigorous manufacturing standards-saving billions annually while maintaining safety and effectiveness.
Categories
- Medication Information (111)
- Health and Wellness (52)
- Women's Health (6)
- Support Resources (5)
- Supplements (5)
- Pharmacy Reviews (5)
- Dermatology (4)
- Mental Health (4)
- Nutrition (3)
- Fitness and Wellness (3)
Archives
- February 2026 (10)
- January 2026 (27)
- December 2025 (30)
- November 2025 (24)
- October 2025 (29)
- September 2025 (14)
- August 2025 (2)
- July 2025 (7)
- June 2025 (2)
- May 2025 (3)
- April 2025 (4)
- March 2025 (3)
- online pharmacy
- dietary supplement
- medication safety
- health benefits
- side effects
- generic drugs
- treatment
- wellness
- optimal health
- diabetes management
- safe medication purchase
- online pharmacy Australia
- brand name drugs
- drug interactions
- authorized generics
- generic medications
- link
- women's health
- dietary supplements
- sleep
