TNF Inhibitors: How Biologics Work for Autoimmune Conditions
 Feb, 8 2026
Feb, 8 2026
When your body turns against itself, inflammation doesn’t just cause pain-it can destroy joints, damage organs, and shut down daily life. For millions with autoimmune conditions like rheumatoid arthritis, psoriasis, or Crohn’s disease, TNF inhibitors have changed everything. These aren’t ordinary drugs. They’re precision-engineered biologics designed to silence one of the body’s most powerful inflammatory signals: tumor necrosis factor alpha (TNFα). But how exactly do they work? And why do some people get life-changing relief while others face side effects or lose response over time?
What TNFα Does in Autoimmune Diseases
TNFα isn’t evil. It’s a natural part of your immune system. Made mostly by immune cells called macrophages, TNFα helps fight infections, triggers fever, and signals other immune cells to attack invaders. But in autoimmune diseases, this system goes rogue. TNFα is produced in excess, turning into a constant alarm bell that keeps the immune system attacking healthy tissue. In rheumatoid arthritis, it erodes joint cartilage. In Crohn’s disease, it tears apart the gut lining. In psoriasis, it speeds up skin cell growth, causing thick, scaly patches. Research shows TNFα sits at the top of a chain reaction. When it binds to receptors (TNFR1 and TNFR2) on cells, it flips switches that release more inflammatory chemicals like IL-1, IL-6, and chemokines. It also makes blood vessels leaky and sticky, letting immune cells flood into tissues where they don’t belong. Without TNFα, this whole cascade collapses. That’s why blocking it works so well.The Five FDA-Approved TNF Inhibitors
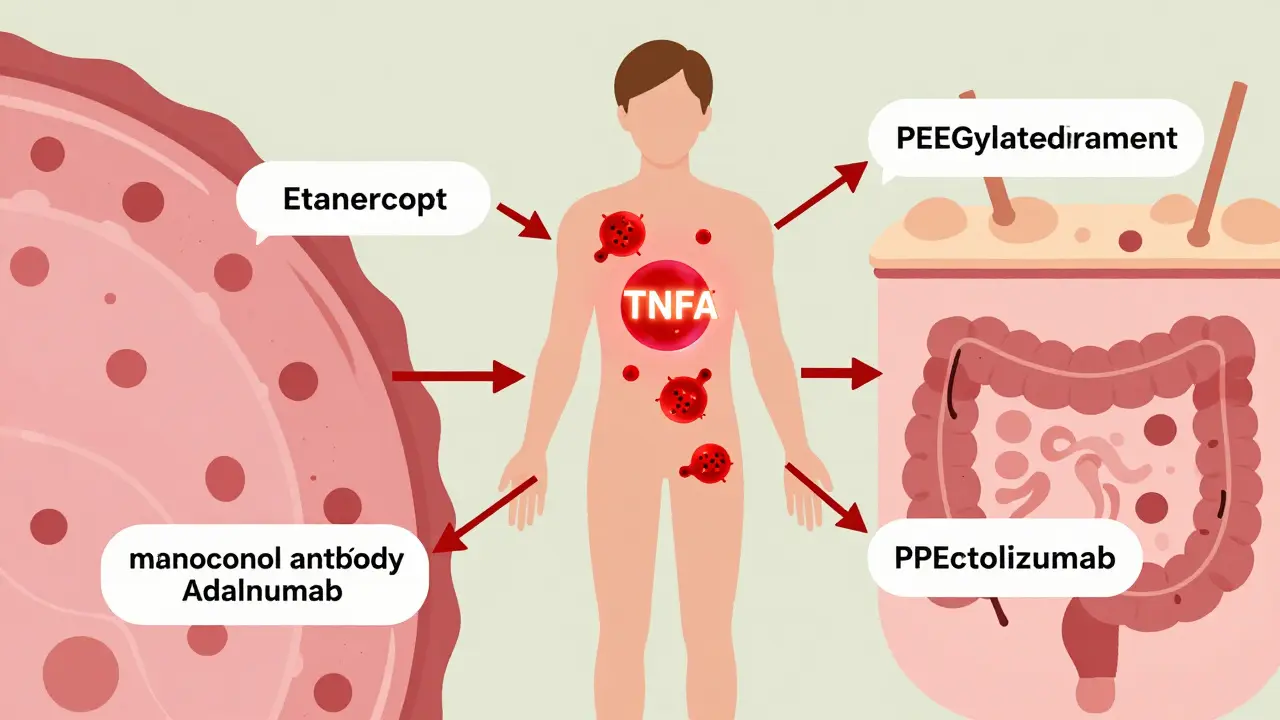
There are five TNF inhibitors approved in the U.S. for autoimmune conditions: etanercept (Enbrel), infliximab (Remicade), adalimumab (Humira), golimumab (Simponi), and certolizumab pegol (Cimzia). They all target TNFα-but they don’t all work the same way. Etanercept is a fusion protein. Think of it as a decoy. It’s built from the outer part of the TNF receptor, attached to a piece of human antibody. It floats in the bloodstream, grabbing onto free TNFα before it can reach real receptors. It mainly blocks soluble TNF, the kind that circulates in blood. The other four-infliximab, adalimumab, golimumab, and certolizumab-are monoclonal antibodies. These are lab-made proteins that lock onto TNFα like a key fits a lock. Infliximab, adalimumab, and golimumab bind to both soluble and membrane-bound TNF. That means they don’t just soak up circulating TNF; they can also attach to TNF on the surface of immune cells, triggering those cells to die. This is called antibody-dependent cell-mediated cytotoxicity (ADCC). Certolizumab is different. It’s a fragment of an antibody, attached to polyethylene glycol (PEG) to make it last longer. It only binds soluble TNF, and it doesn’t trigger cell death.How TNF Inhibitors Actually Stop Inflammation
When TNFα is blocked, the whole inflammatory system slows down. Less IL-6 means fewer fever spikes and less joint swelling. Less IL-8 and RANTES means fewer immune cells rushing to the wrong places. Fewer adhesion molecules like ICAM-1 mean white blood cells can’t cling to blood vessel walls to sneak into tissues. Over time, this stops tissue damage. But it’s not just about blocking TNF. Studies show these drugs do more:- They can trigger apoptosis-programmed cell death-in overactive immune cells.
- They reduce oxidative stress markers in the blood.
- They change how monocytes behave, lowering the production of other inflammatory signals.
Why Some People Stop Responding
Not everyone responds. And not everyone stays responsive. Around 30-40% of patients experience what’s called secondary failure: the drug works at first, then loses its effect. Why? Your immune system sometimes sees the biologic as a foreign invader. It builds antibodies against it-anti-drug antibodies. These antibodies bind to the drug, tag it for destruction, and clear it from your blood before it can do its job. This is more common with infliximab and adalimumab because they’re fully human proteins. Etanercept, being a fusion protein, is less likely to trigger this reaction. It can take years. A patient might use Humira for five years with perfect control, then suddenly notice their joints stiffening again. Blood tests can check for anti-drug antibodies. Sometimes, switching to another TNF inhibitor helps. Other times, you need to move to a different class of biologic entirely.Risks and Side Effects
TNF inhibitors are powerful, but they come with serious risks. Because they suppress part of your immune system, you’re more vulnerable to infections. The risk of serious infections like tuberculosis, pneumonia, or fungal infections is 2-5 times higher than in the general population. That’s why doctors test for latent TB before starting treatment. A simple skin or blood test can catch hidden TB. If it’s there, you get antibiotics first. Injection site reactions are common with subcutaneous drugs like adalimumab and etanercept. About 20-30% of users report redness, itching, or swelling where they inject. Most fade within a day or two. There’s also a strange paradox: in rare cases, TNF inhibitors can trigger new inflammatory conditions. Some patients develop psoriasis, lupus-like symptoms, or even neurological issues like multiple sclerosis. Why? One theory is that TNF inhibitors can’t cross the blood-brain barrier. So while they calm inflammation in the body, they might leave the brain exposed to unbalanced signals. TNFR1 and TNFR2 have different roles-blocking one might accidentally overactivate the other.
How Treatment Works in Practice
TNF inhibitors aren’t pills. They’re either injected under the skin or given through an IV. Subcutaneous versions (etanercept, adalimumab, golimumab, certolizumab) are usually self-administered at home. Most people learn to inject themselves in 1-2 weeks. Infliximab requires a clinic visit every 4-8 weeks for a 1-2 hour infusion. Doctors start with a low dose and gradually increase if needed. It takes weeks to months to see full results. Many patients feel better in 4-6 weeks, but the real benefit-slowing joint damage-shows up over 6-12 months. Manufacturers offer support programs. AbbVie’s Humira Complete gives patients 24/7 nurse support, injection training, and help with insurance. Janssen’s Inflectra Connect does the same for Remicade. These programs aren’t just helpful-they’re essential for long-term success.The Market and What’s Next
In 2022, TNF inhibitors made up about $35 billion of the global biologics market. Humira alone brought in $21.2 billion before biosimilars arrived. Now, cheaper biosimilar versions (like Amjevita for Humira) are cutting costs and expanding access. But the future isn’t just more TNF inhibitors. Newer biologics targeting IL-17 and IL-23 are showing better results for psoriasis and psoriatic arthritis. Some patients are switching. Still, TNF inhibitors remain the most studied and widely used biologics for rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. Research is now focused on smarter drugs: ones that block TNFR1 (the bad actor in inflammation) while leaving TNFR2 (which helps repair tissue) alone. Early trials are promising. The goal isn’t just to suppress inflammation-it’s to restore balance.Real Patient Experiences
One woman in Sydney, diagnosed with rheumatoid arthritis at 32, went from needing a walker to hiking 5 miles a week after six months on adalimumab. Another man with Crohn’s disease went from 12 bowel movements a day to none after starting infliximab. But it’s not all success stories. One Reddit user described spending $1,200 out-of-pocket every month before insurance kicked in. Another said their skin broke out in psoriasis after switching from etanercept to adalimumab. The truth? TNF inhibitors work miracles for some. For others, they’re a temporary fix-or a dead end. Success depends on your disease, your body, and how well you and your doctor manage the risks.How long does it take for TNF inhibitors to work?
Most people start noticing symptom relief within 4 to 6 weeks, but full benefits-like reduced joint damage or healing of intestinal lining-can take 3 to 6 months. Unlike painkillers that act fast, TNF inhibitors work by changing the underlying disease process, which takes time.
Can you take TNF inhibitors with other medications?
Yes, but carefully. TNF inhibitors are often combined with methotrexate, which helps reduce the chance of developing anti-drug antibodies. Avoid live vaccines while on treatment. Always tell your doctor about all supplements, over-the-counter drugs, and herbal remedies-some can increase infection risk or interfere with drug metabolism.
Do TNF inhibitors cause weight gain?
Weight gain isn’t a direct side effect of TNF inhibitors. But some patients gain weight because their disease improves-less pain means more movement, better appetite, and improved energy. Others may gain due to steroid use before starting biologics. It’s not the drug itself, but the body’s recovery.
Are TNF inhibitors safe during pregnancy?
Some TNF inhibitors, like adalimumab and certolizumab, are considered low-risk during pregnancy and are often continued to keep disease under control. Etanercept and infliximab are also used, but with more caution. Stopping treatment can lead to disease flare-ups, which are riskier for pregnancy than the drug. Always consult a rheumatologist or OB-GYN who specializes in autoimmune conditions.
What happens if I stop taking my TNF inhibitor?
Stopping can cause your autoimmune disease to flare back quickly-sometimes within weeks. Symptoms like joint pain, fatigue, or diarrhea can return worse than before. If you need to stop (due to infection, surgery, or side effects), your doctor will likely start you on a different medication first to avoid a rebound effect.

Joseph Charles Colin
February 9, 2026 AT 13:57TNFα inhibition is a master switch in the cytokine cascade-blocking it doesn't just dampen inflammation, it dismantles the entire signaling architecture that sustains autoimmune pathology. The key nuance is differential binding: etanercept's decoy receptor architecture preferentially neutralizes soluble TNF, while monoclonal antibodies like infliximab and adalimumab engage membrane-bound TNF, triggering ADCC and apoptosis of activated immune cells. This distinction explains differential efficacy in Crohn’s versus RA-membrane TNF is dominant in gut mucosal inflammation, hence the superior mucosal healing seen with antibody-based agents.
Moreover, the pharmacokinetics of PEGylated certolizumab reduce immunogenicity and placental transfer, making it the preferred biologic in pregnancy. But the elephant in the room? Anti-drug antibody formation. Non-humanized Fc regions in infliximab and adalimumab are immunogenic hotspots. Co-administration with methotrexate isn't just helpful-it's essential for sustaining drug levels. Without it, ADA titers spike, clearance accelerates, and you get secondary failure.
And don't get me started on biosimilars. Amjevita isn't a 'generic'-it's a structurally identical molecule with identical pharmacodynamics. The price drop isn't charity; it's market correction. Pharma's patent evergreening tactics are unethical, not innovative.
Joshua Smith
February 10, 2026 AT 15:11This was really helpful, thanks for breaking it down. I’ve been on Humira for three years and was curious about why my doctor keeps mentioning methotrexate. Never realized it was to stop my body from attacking the drug itself. Makes sense now.
Chima Ifeanyi
February 11, 2026 AT 09:46Let me be blunt: TNF inhibitors are a $35 billion placebo machine fueled by pharma greed. You think blocking TNFα is precision medicine? It’s a sledgehammer. The fact that 40% of patients lose response proves it’s not targeting the root cause-it’s suppressing symptoms while the real drivers (mitochondrial dysfunction, gut dysbiosis, epigenetic dysregulation) fester unchecked. And don’t even get me started on the TB risk. They screen for latent TB but ignore the fact that chronic TNF blockade reactivates latent herpesviruses like EBV and HHV-6, which are now linked to MS and lymphoma. This isn’t treatment. It’s immunological roulette.
Also, why are we still using these 20-year-old drugs when IL-23 inhibitors like risankizumab show 70%+ PASI-90 response in psoriasis? Because the FDA and AMA are in bed with AbbVie. Wake up.
Ritteka Goyal
February 11, 2026 AT 10:10OMG I just read this and I’m so emotional!! I have RA and I started on Enbrel in 2020 and now I can play with my kids without crying from pain!! I mean, before I couldn’t even hold my coffee cup!! But I think America is so behind in biologics!! In India we have better access to biosimilars and my cousin in Mumbai got Humira for 1/10th the price!! Why is the US so expensive?? Also I think TNF is not the only problem!! I think gut microbiome is the real culprit!! My doctor in Delhi said 80% of autoimmune disease starts in the gut!! So why aren’t we doing fecal transplants instead of these expensive drugs??
And also I read on Reddit that some people get psoriasis from TNF inhibitors!! I think this is because they are not testing for HLA-B27 before prescribing!! I think they should test everyone for HLA-B27 and also for MTHFR mutations!!
Also I think the FDA should ban all TNF inhibitors because they are too dangerous!! My friend’s brother got lymphoma after 2 years!!
Frank Baumann
February 13, 2026 AT 03:21I’ve been on this rollercoaster for 12 years. First, methotrexate-nope. Then Enbrel-worked for two years, then poof. Humira? Same thing. Then Remicade-gave me a fever that lasted 11 days. I’ve had three infections that landed me in the hospital. I’ve lost jobs. I’ve lost friends. I’ve lost sleep. And now? Now I’m told to switch to a JAK inhibitor? What’s next? A bone marrow transplant? They don’t tell you this when you sign the consent form. They say ‘life-changing.’ They don’t say ‘you’re now a walking immunosuppressed target.’
My wife says I’m lucky. Lucky? I’m lucky I haven’t died yet.
Ken Cooper
February 13, 2026 AT 21:41hey so i just wanted to say this post was super helpful!! i had no idea that certolizumab was the only one that doesn't trigger cell death!! that's wild!! and also i didn't know that methotrexate helps with anti-drug antibodies!! my doc just said 'take this too' and never explained why!!
also i got my first shot last week and my arm was sore for 3 days but it's fine now!! i'm kinda scared of the infection thing tho... i have a toddler and i'm always worried about germs!! any tips??
Andy Cortez
February 15, 2026 AT 19:22Oh wow. So TNF inhibitors are just fancy immunosuppressants? I thought they were magic. Turns out they’re just expensive Band-Aids on a bullet wound. And let’s be real-people who say ‘it changed my life’ are the ones who didn’t have to pay $1,200/month out of pocket. Meanwhile, my cousin in Ohio got denied insurance coverage because her RA wasn’t ‘severe enough.’
And what’s with the ‘paradoxical psoriasis’? Sounds like the drug is trying to kill itself. Like a self-sabotaging AI. Also, why is the FDA still approving these when we have CRISPR trials for gene editing in autoimmune diseases? Because Big Pharma doesn’t want cures. They want lifelong subscriptions.
Andrew Jackson
February 16, 2026 AT 21:57It is with profound moral concern that I address this matter. The pharmaceutical industry, under the guise of scientific advancement, has orchestrated a systemic erosion of bodily autonomy under the banner of 'biologic therapy.' To artificially suppress a fundamental immunological pathway-TNFα-is not healing; it is hubris. Nature did not design the immune system to be silenced. It was designed to be balanced. To intervene with monoclonal antibodies is to usurp the divine order of physiological homeostasis. One must ask: Who gave man the right to reengineer the very language of inflammation? And if we silence TNFα today, what cascade of unintended consequences will emerge tomorrow? The body is not a machine to be patched. It is a temple. And we are becoming its sacrilegious custodians.
Patrick Jarillon
February 18, 2026 AT 09:54Of course they’re saying TNF is the problem. That’s what the FDA, WHO, and Big Pharma want you to believe. But here’s the truth: TNFα doesn’t cause autoimmune disease. It’s a biomarker. The real culprit? 5G radiation. It disrupts your mitochondrial membrane potential, which causes macrophages to overproduce TNF as a stress response. They don’t tell you this because 5G is owned by defense contractors who also own the patent rights to Humira. And the TB screening? That’s a cover. The real danger is latent Epstein-Barr virus reactivation triggered by PEGylated compounds. That’s why you see lymphomas in 3-5 year users. They just call it ‘rare adverse event.’
And biosimilars? They’re not equivalent. The PEG chain length varies by manufacturer. It’s not regulated. You’re basically playing Russian roulette with your immune system.
Kathryn Lenn
February 20, 2026 AT 03:45Wow. So TNF inhibitors are basically putting a bandaid on a nuclear reactor. Congrats, science. You’ve created a drug that works… until it doesn’t. And then you’re stuck with a broken immune system and a $15,000 monthly bill. And let’s not forget the fun part: developing psoriasis because your body’s trying to compensate. I’m just waiting for the next headline: ‘Woman develops werewolf syndrome after Humira.’
Also, why do they call it ‘precision medicine’ when it’s basically a shotgun blast to the immune system? Precision would be fixing the root cause. Not silencing the alarm bell.
Elan Ricarte
February 20, 2026 AT 17:23Let me tell you what they don’t tell you in the brochures: TNF inhibitors are the opioid of autoimmune care. You start feeling amazing, so you keep taking it. Then you need more. Then you get addicted to the relief. Then you crash. And when you crash? You’re left with a body that’s forgotten how to self-regulate. The drug didn’t fix your immune system-it hijacked it. And now? You’re dependent on a biologic that costs more than your car. Meanwhile, the real problem-your leaky gut, your endocrine chaos, your chronic stress-is still there, festering under the surface. We’re not treating disease. We’re treating symptoms with a sledgehammer and calling it innovation. Pathetic.
Angie Datuin
February 22, 2026 AT 00:38Thank you for writing this. I’ve been on adalimumab for five years. It’s not perfect, but it let me keep working. I didn’t know about the ADCC mechanism-now I understand why my doctor switched me from etanercept. It’s comforting to know it’s not just ‘magic.’
Camille Hall
February 23, 2026 AT 12:02This is such a clear breakdown. I’ve been trying to explain this to my sister who has Crohn’s, and she kept thinking biologics were ‘chemo.’ Now I can send her this. Also, the part about certolizumab and pregnancy? That’s huge. So many patients are terrified to get pregnant-this info could change lives.
Monica Warnick
February 23, 2026 AT 18:42So… I just found out I have anti-drug antibodies? After 7 years on Humira? I thought I was ‘cured.’ Turns out I was just on a delay. Now I’m switching to a JAK inhibitor. I’m scared. What if it doesn’t work? What if I get cancer? What if I can’t afford it? I’m 34. I have two kids. I can’t go back to pain. I can’t go back to being that person who cried in the bathroom because she couldn’t hold her coffee. Please… someone tell me it’s going to be okay.
Jonah Mann
February 25, 2026 AT 16:53yo i just had a question-when they say 'TNFα isn't evil,' does that mean it's kinda like a good guy gone bad? like a villain origin story? also, is it true that some people have 'TNFα hypersensitivity' and that's why they react worse? my doc said something about SNPs in the TNFR1 gene… i didn't get it but i think i'm one of those people?
Joseph Charles Colin
February 25, 2026 AT 18:27Re: comment from 7568-yes, there are TNFR1 polymorphisms (rs767455, rs1800693) associated with hyperresponsiveness to TNFα signaling. These are linked to more aggressive disease phenotypes and higher rates of secondary failure. But here’s the kicker: patients with these SNPs often respond better to TNFR1-selective inhibitors currently in phase 2 trials. That’s the future: not broad TNF blockade, but precision targeting of the pathological receptor subtype. We’re moving from shotgun to sniper rifle.