Hemophilia Causes, Symptoms & Diagnosis Guide
 Oct, 23 2025
Oct, 23 2025
Hemophilia Bleeding Risk Calculator
Factor Level Calculator
Enter your Factor VIII or IX activity level to see your bleeding risk and treatment recommendations.
Your Bleeding Risk Assessment
Risk Level: Not calculated yet
Treatment Recommendations
Select your factor level to see recommendations
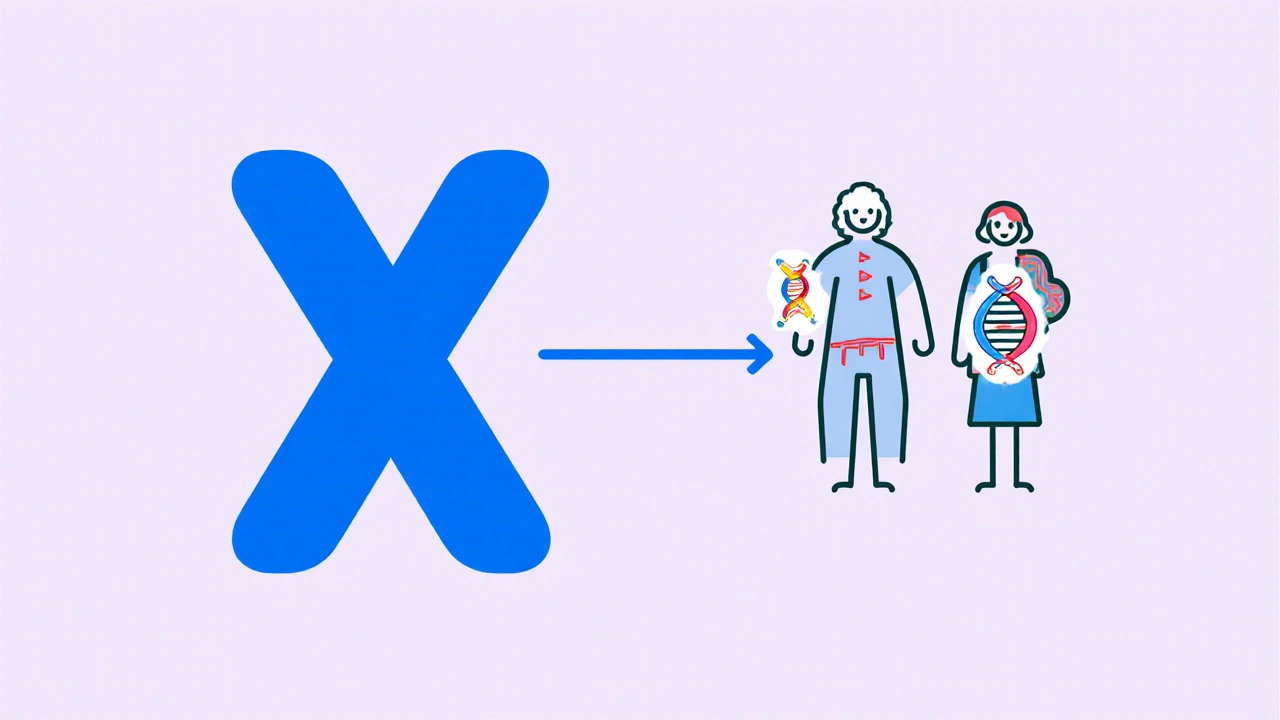
When discussing Hemophilia is a genetic bleeding disorder caused by deficiency of clotting factors, the first thing to know is that it isn’t a single disease but a group of related conditions. The most common forms, Hemophilia A and Hemophilia B, stem from missing or malfunctioning Factor VIII and Factor IX respectively. Understanding why the body can’t form a stable clot helps make sense of the bleeding patterns you’ll read about later.
Genetic roots - why hemophilia happens
- X‑linked recessive inheritance: The genes for Factor VIII (F8) and Factor IX (F9) sit on the X chromosome. Men have one X, so a single defective copy triggers the disease. Women typically carry one mutated copy and are carriers.
- Gene mutations: Over 3,000 distinct mutations have been catalogued for F8 and about 1,200 for F9. Large deletions, point mutations, and inversions each affect how much functional protein is produced.
- De‑novo mutations: About one‑third of cases arise from a new mutation in the mother’s egg cell, meaning there’s no family history.
- Acquired hemophilia: Rarely, the immune system creates antibodies (inhibitors) that neutralize Factor VIII, leading to a sudden bleeding tendency in adults with no genetic link.
Typical symptoms - what to watch for
- Prolonged bleeding after minor cuts or dental work.
- Spontaneous bruising, especially on the arms and legs.
- Hemarthrosis - bleeding into joints such as the knee, elbow, or ankle. This causes swelling, warmth, and limited movement.
- Deep tissue bleeds that can create large hematomas, sometimes visible as a bulge under the skin.
- Bleeding after vaccinations or circumcisions, which can be unusually prolonged.
Joint bleeds are the hallmark of severe hemophilia. Repeated hemarthrosis leads to chronic synovitis and eventually joint arthropathy, an irreversible condition that mimics osteoarthritis.
How doctors confirm the diagnosis
The diagnostic pathway combines basic screening tests, specific factor assays, and, when needed, genetic analysis.
- Prothrombin time (PT): Usually normal because the extrinsic pathway remains intact.
- Activated partial thromboplastin time (aPTT): Typically prolonged, reflecting a problem in the intrinsic pathway where Factors VIII and IX act.
- Factor activity assay: Quantifies the exact percentage of functional Factor VIII or IX. Levels below 1% denote severe disease, 1‑5% moderate, and 5‑40% mild.
- Inhibitor testing: A Bethesda assay measures antibodies that may neutralize replacement therapy.
- Genetic testing: DNA sequencing of F8 and F9 identifies the precise mutation, guiding family counseling and prenatal options.
- Imaging: Ultrasound is the first line for detecting hemarthrosis; MRI can assess chronic joint damage.
Because early detection can prevent irreversible joint damage, many pediatric hemophilia centers recommend routine screening for newborns with a family history.

Quick reference: Hemophilia A vs. Hemophilia B
| Type | Deficient Factor | Typical Severity Distribution | Inheritance Pattern | Common Mutations |
|---|---|---|---|---|
| Hemophilia A | Factor VIII | Severe ~40%, Moderate ~30%, Mild ~30% | X‑linked recessive | Intron 22 inversion, point mutations |
| Hemophilia B | Factor IX | Severe ~45%, Moderate ~35%, Mild ~20% | X‑linked recessive | Large deletions, missense mutations |
Checklist for patients and caregivers
- Know your exact factor level - keep a copy of the latest lab report.
- Carry a medical alert card that lists the specific type (A or B) and any known inhibitors.
- Schedule regular orthopedic check‑ups when joint bleeds occur.
- Maintain a bleeding diary: date, location, trigger, treatment used.
- Discuss genetic counseling with a specialist if you plan future pregnancies.
Common pitfalls and how to avoid them
Even seasoned clinicians can miss subtle signs. Here are three frequent errors and practical ways to sidestep them:
- Assuming normal PT rules out a clotting disorder - always order an aPTT if bleeding is unexplained.
- Overlooking mild hemophilia - patients with >5% activity may still bleed after surgery; obtain a factor assay before any invasive procedure.
- Neglecting inhibitor testing - a missed inhibitor can turn standard factor replacement into a failed therapy, leading to dangerous delayed bleeding.
Next steps after a diagnosis
Once hemophilia is confirmed, the care plan typically involves three pillars: prophylactic factor replacement, joint health monitoring, and family education.
- Prophylaxis: Regular infusions of clotting factor concentrate keep levels above 1% and dramatically cut joint bleeds.
- Joint surveillance: Use ultrasound every 6‑12 months for children; MRI for adults with persistent pain.
- Family outreach: Offer carrier testing for mothers and sisters, and discuss prenatal diagnostics such as chorionic villus sampling.
With modern recombinant products and gene‑therapy trials, the outlook for people with hemophilia is steadily improving.
Frequently Asked Questions
What is the difference between hemophilia A and B?
Hemophilia A is caused by a deficiency of Factor VIII, while hemophilia B results from low Factor IX. Both follow an X‑linked recessive pattern, but the specific genetic mutations differ, which can affect treatment response.
Can women have hemophilia?
Women are usually carriers, but about 5‑10% of female carriers develop symptoms because of skewed X‑chromosome inactivation or rare homozygous mutations.
How is hemophilia diagnosed in newborns?
If there’s a known family history, doctors can order a coagulation screen (aPTT) within the first weeks of life. A definitive diagnosis uses a factor activity assay and, when possible, genetic testing.
What are inhibitors and why are they a problem?
Inhibitors are antibodies that neutralize infused clotting factors. They render standard replacement therapy ineffective, requiring bypass agents or immune tolerance induction, which are more complex and costly.
Is gene therapy available for hemophilia?
Clinical trials in 2024‑2025 have shown that a single infusion of an adeno‑associated virus vector can raise Factor VIII or IX levels to the mild range for years, but regulatory approval is still pending in most regions.

Kristin Violette
October 23, 2025 AT 22:08Great overview! The distinction between Factor VIII and IX mutations really underscores why genetic counseling is non‑negotiable. When you mention the intron‑22 inversion, that's a classic example of a hotspot that clinicians use to predict inhibitor risk. I always tell families to keep a digital copy of the sequencing report-makes future therapy decisions smoother. Bottom line: understanding the molecular underpinnings translates directly into personalized prophylaxis.
Theo Asase
October 24, 2025 AT 06:26Don't be fooled by the glossy pharma press releases; they're hiding the fact that the real cure is being suppressed. The same labs that churn out recombinant factors are funded by shadowy lobbyists who profit from lifelong infusions. You think gene therapy is 'pending approval'? Think again-there's a whole cabal of executives keeping it under lock and key. Wake up, people!
Joey Yap
October 24, 2025 AT 17:33Reading through the guide reminded me how fragile our clotting balance truly is, a reminder of the body's intricate self‑regulation. It’s heartbreaking to see families wrestling with the uncertainty of inhibitor development, yet also uplifting to witness the community’s resilience. I appreciate how the author highlighted both the science and the lived experience. May we keep centering patient voices as we push forward.
Lisa Franceschi
October 25, 2025 AT 03:16While I understand concerns about pharmaceutical practices, the evidence supporting recombinant factor safety is robust and peer‑reviewed. Regulatory agencies worldwide have stringent approval pathways, which include extensive phase III trials. It's important to separate legitimate skepticism from unfounded conspiracy narratives that can erode patient trust. Collaborative dialogue, grounded in data, will ultimately serve the hemophilia community best.
Shermaine Davis
October 25, 2025 AT 10:13Its awsome how much info i learned!
Nicholai Battistino
October 26, 2025 AT 04:40Hemophilia management has come a long way since the days of whole blood transfusions. Modern therapies now include recombinant factor concentrates that are virus‑free. Prophylactic regimens aim to keep trough factor levels above 1 % to prevent spontaneous bleeds. Early initiation of prophylaxis in childhood is associated with reduced joint arthropathy. Regular joint imaging, especially ultrasound, helps detect subclinical hemarthroses. Families should maintain a bleeding diary to track triggers and treatment responses. Genetic testing not only confirms the diagnosis but also informs carrier status for relatives. Inhibitor development remains a significant complication, occurring in roughly 30 % of severe cases. When inhibitors are present, bypass agents such as activated prothrombin complex concentrate become necessary. Immune tolerance induction protocols can eradicate inhibitors in many patients. Emerging gene‑therapy trials have shown sustained factor expression for several years. However, long‑term safety data are still accumulating, and eligibility criteria are strict. Multidisciplinary care teams, including hematologists, physiotherapists, and psychologists, optimize outcomes. Education of school personnel and emergency responders is crucial for safe day‑to‑day living. Ultimately, a combination of medical advances and patient empowerment drives better quality of life.
Aimee White
October 26, 2025 AT 14:23Wow, those 15 sentences just blew the roof off the old‑school narrative! The gene‑therapy hype isn’t just science fiction; it’s a covert operation to keep the old money flowing from endless factor purchases. Those trial participants are like guinea‑pigs in a lab‑rat circus, and the public never gets the full story. You can’t ignore the ethical quagmire when a single infusion could replace a lifetime of infusions-yet the big players keep it “experimental.”
Javier Muniz
October 26, 2025 AT 18:33Hey, love the energy! Honestly, the data on gene‑therapy looks promising, but we still need long‑term follow‑up before we call it a miracle. Until then, sticking with proven prophylaxis and good joint care is the safe bet. If anyone’s curious about trial eligibility, I’ve got a few resources to share. Keep the convo going!
Andrew Wilson
October 27, 2025 AT 08:26People need to step up and support the hemophilia community, not just sit on the sidelines. It's a moral failure when we let greed dictate access to lifesaving treatment. We should demand fair pricing and universal coverage for factor replacement. Anything less is just plain wrong.
Diane Larson
October 27, 2025 AT 16:46Absolutely, Andrew-price gouging is unacceptable, and advocacy can make a difference. Organizations like the World Federation of Hemophilia lobby for better reimbursement policies worldwide. You can also join local patient groups that lobby state legislators for mandated coverage. Together we can push for transparent pricing and equitable access.
Michael Kusold
October 27, 2025 AT 22:20Just a heads‑up: staying on top of your factor levels and keeping a medical ID bracelet can save you a lot of trouble down the road.