Drug Interactions Discovered Post-Market: What It Means for Your Safety
 Dec, 3 2025
Dec, 3 2025
Most people assume that if a drug is approved and on the shelf, it’s been thoroughly tested for safety. But here’s the truth: drug interactions that can cause serious harm - even death - are often found after millions of people have already taken the medicine. This isn’t a flaw in the system. It’s how the system is designed to work.
Why Clinical Trials Miss Dangerous Interactions
Before a drug hits the market, it goes through clinical trials. These studies usually involve 1,000 to 5,000 people over 6 to 12 months. They’re tightly controlled. Participants are generally healthy, younger, and taking only one or two medications. They’re monitored closely. But real life? It’s messy. Real patients are older. They have diabetes, kidney disease, heart failure. They’re on five or six prescriptions. They drink grapefruit juice with breakfast. They take St. John’s Wort for low mood. They skip doses or double up when they feel worse. None of that shows up in trials. That’s why the FDA found that nearly one-third of new drugs approved between 2001 and 2011 had a major safety event after they were sold - a black box warning, a recall, or a public alert. These weren’t rare flukes. They were predictable blind spots.How Dangerous Interactions Get Found
Once a drug is out in the wild, millions of people start using it. That’s when the real data starts pouring in. Doctors, pharmacists, and patients report strange side effects - muscle pain, dizziness, bleeding, irregular heartbeat - that don’t match the label. These reports go into systems like the FDA’s FAERS (FDA Adverse Event Reporting System) and the EU’s EudraVigilance. These aren’t just random complaints. They’re analyzed using AI and statistical models. For example, if 200 people taking simvastatin and fluconazole (an antifungal) all show up in the system with severe muscle damage, the system flags it. That’s how the interaction between these two drugs was confirmed - and why doctors now warn patients: don’t mix them. One of the most dangerous mechanisms involves the CYP3A4 enzyme. This enzyme in your liver breaks down many drugs. If another drug blocks it - like grapefruit juice, ketoconazole, or even some antibiotics - the first drug builds up to toxic levels. Simvastatin with fluconazole? Blood levels can spike 3 to 10 times. Grapefruit juice with atorvastatin? Up to 15 times higher. That’s not a side effect. That’s a chemical explosion waiting to happen.Real Cases That Changed Medicine
Terfenadine (Seldane), a popular antihistamine, was pulled from the market after it was found to cause fatal heart rhythms when taken with ketoconazole or erythromycin. Patients didn’t know. Their doctors didn’t know. The interaction wasn’t visible in trials because those drugs weren’t tested together. Benfluorex (Mediator), a weight-loss drug, was used by over 5 million people in France over 30 years. Then, doctors noticed a pattern: patients were developing rare heart valve damage. By the time it was banned in 2009, thousands had suffered permanent injury. Even newer drugs aren’t safe. In 2012, the FDA issued a warning about Exalgo, an extended-release painkiller. It was found that drinking alcohol with it caused the pill to release its full dose at once - leading to overdose and death. That interaction was missed because trials didn’t include people who drank regularly.
Who Reports These Interactions - And Why Most Go Unreported
Only about 5% to 10% of serious adverse events are ever reported. Why? Doctors are busy. Patients don’t connect the dots. They think the nausea is from stress, the muscle pain is from aging. Pharmacists might catch it - if they’re asked. Reddit threads from pharmacy communities are full of stories like this: “My doctor never warned me about grapefruit and Lipitor. I ended up in the ER with kidney damage.” Or: “I took ciprofloxacin with my blood pressure med. My pharmacist stopped me - said it could cause a deadly heart rhythm. Saved my life.” The FDA’s FAERS database has over 2,800 reports of rhabdomyolysis (muscle breakdown) from statin interactions alone. Simvastatin with antifungals makes up nearly 40% of those cases. These aren’t hypotheticals. They’re real people, in real hospitals, because a warning was missing.How the System Is Fixing Itself
The FDA didn’t wait for more deaths. In 2008, they launched the Sentinel Initiative - a network that monitors 300 million patient records across hospitals and insurers. It’s like a national alarm system for drug safety. If a spike in kidney failure pops up in a certain region after a new drug is prescribed, they can trace it back within weeks. In 2023, the FDA approved the first AI-powered pharmacovigilance tool that can scan 10,000 adverse event reports a day with 92.7% accuracy. The European Medicines Agency cut signal detection time from 18 months to 45 days using similar tech. Pharmacists now use tools like the Naranjo Algorithm to assess whether a reaction is likely caused by a drug interaction. It’s not perfect, but it’s better than guessing. And apps like GoodRx now include interaction warnings - not because they’re required, but because patients demand it.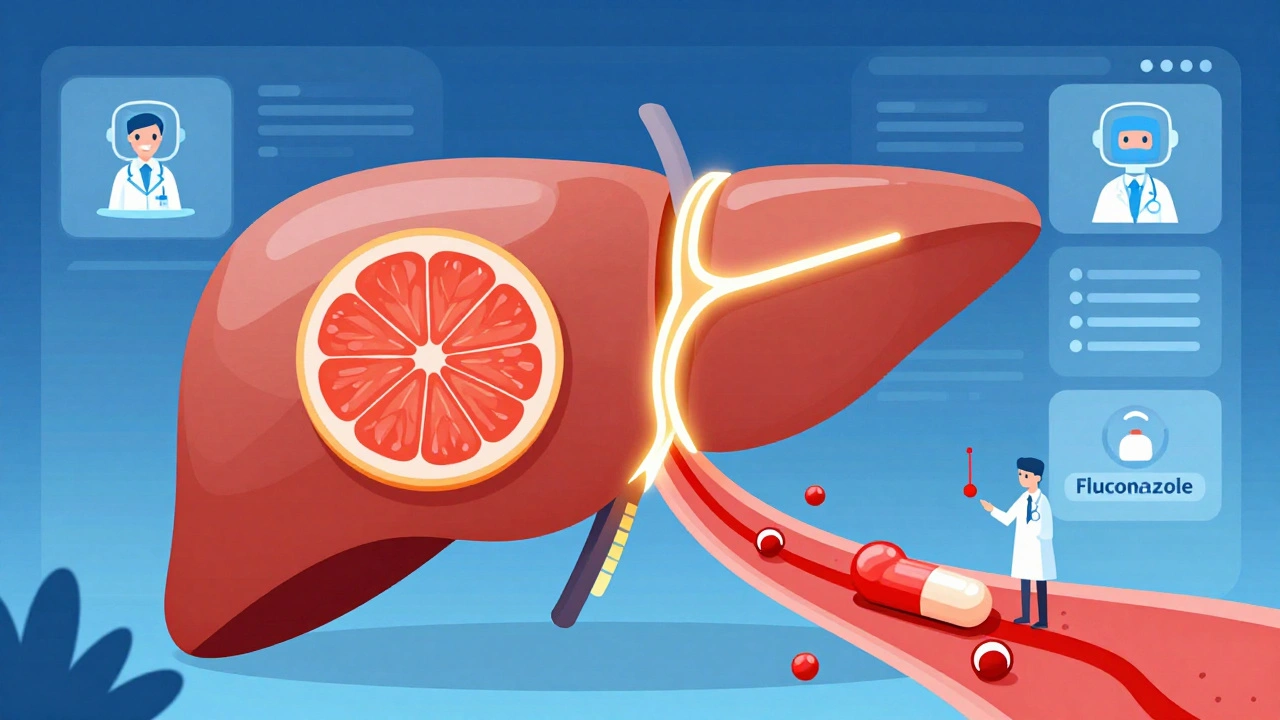
What You Can Do to Stay Safe
You don’t need to be a scientist to protect yourself. Here’s what actually works:- Always tell your doctor and pharmacist every medication you take - including vitamins, herbs, and over-the-counter pills.
- Ask: “Could this interact with anything else I’m on?” Don’t assume they already know.
- Use free tools like GoodRx or Medscape’s drug interaction checker. They’re not perfect, but they catch the big ones.
- If you start a new drug and feel something weird - muscle pain, unusual fatigue, dizziness, heart palpitations - don’t ignore it. Call your pharmacist. They’re trained to spot this stuff.
- Know your triggers. Grapefruit juice? Avoid it if you’re on statins, blood pressure meds, or some antidepressants. Alcohol? Don’t mix it with opioids, benzodiazepines, or certain antibiotics.

Rebecca Braatz
December 5, 2025 AT 09:44Y’all need to stop treating your meds like candy. I’m a pharmacist and I see this every single day-people take five different pills, toss in some turmeric supplement, drink grapefruit juice like it’s water, then wonder why they feel like crap. It’s not magic, it’s pharmacology. Talk to your pharmacist. Seriously. We’re not just the people who hand out pills-we’re your safety net.
And yes, I know doctors are busy. But if you don’t speak up, who will?
Stop being passive. Your life isn’t a gamble.
Benjamin Sedler
December 5, 2025 AT 18:38Oh wow, another ‘big pharma is evil’ sob story. Let me guess-the FDA is just sitting on their hands waiting for people to drop dead? Newsflash: if drugs were tested for every possible combo with every possible lifestyle choice, we’d still be using leeches in 2024. The system isn’t broken-it’s adaptive. You think they’re gonna test simvastatin with every herbal tea, kombucha, and moon juice on Earth? That’s not science. That’s paranoia with a spreadsheet.
zac grant
December 6, 2025 AT 16:41From a clinical pharmacology standpoint, the post nails the core issue: pharmacovigilance is inherently post-marketing because pre-market trials are underpowered for polypharmacy and real-world variability. The CYP3A4 pathway is the most clinically significant enzyme in drug metabolism-its inhibition is responsible for ~30% of all serious ADRs. The Sentinel Initiative’s use of electronic health record mining has reduced signal detection latency by over 60% since 2015. What’s missing is patient-facing transparency. Most patients don’t know that FAERS is public, or that they can file a report themselves. That’s the real gap.
Also, the Naranjo scale’s sensitivity is only ~78% for drug interactions. We need better AI-driven phenotyping. We’re getting there.
Carolyn Ford
December 6, 2025 AT 19:56Don’t trust the system. Trust yourself. And stop taking anything you don’t absolutely need.
Heidi Thomas
December 7, 2025 AT 04:39Libby Rees
December 7, 2025 AT 23:25It is important to recognize that drug safety is not an event, but a process. Clinical trials serve a necessary function in establishing initial safety profiles. However, the complexity of human physiology, combined with real-world medication use, inevitably introduces variables that cannot be fully replicated in controlled environments. The evolution of post-market surveillance systems, such as FAERS and Sentinel, represents a critical advancement in public health. Patient vigilance remains indispensable. Communication between patients and healthcare providers is not optional-it is foundational to safety.
Rudy Van den Boogaert
December 8, 2025 AT 13:00I’ve been on a statin for 8 years. My doctor never mentioned grapefruit. I found out from a Reddit thread. I stopped drinking it. No issues since. I’m not a doctor, but I’m smart enough to listen when someone says ‘don’t mix this.’
Also, I told my mom about the fluconazole-simvastatin thing after she got hospitalized. She’s 72. She didn’t even know she was on two meds that could kill her. She just trusted the script.
Don’t assume. Ask. Again. And again.
Rachel Bonaparte
December 8, 2025 AT 22:54Let’s be real. The FDA doesn’t care about you. They care about stock prices. Did you know that the same people who approve drugs also sit on pharma boards? It’s all connected. The ‘AI tools’ they brag about? They’re trained on data that’s been scrubbed to avoid liability. The real interactions-the ones that kill-are buried under layers of corporate jargon.
And don’t get me started on ‘free tools’ like GoodRx. They’re sponsored by the same companies that make the drugs. They show you the safe combos… but only the ones that don’t hurt profits.
They’re watching you. They know you’re reading this. And they’re still selling it.
Stay off the grid. Eat clean. Take nothing unless you grew it yourself. Or at least, don’t trust a single pill from a corporation that answers to shareholders, not your heartbeat.
Scott van Haastrecht
December 10, 2025 AT 09:06Another ‘you’re all idiots for not reading the label’ lecture. Newsflash: most people can’t read a medical journal. They’re not doctors. They’re not pharmacists. They’re working 60-hour weeks, raising kids, and trying not to die of stress.
And now you want them to memorize CYP3A4 enzyme pathways? You think they care about statin interactions when they’re choosing between insulin and rent?
This isn’t about ‘personal responsibility.’ It’s about systemic neglect. Pharma makes billions. Patients die. The system rewards silence. And you? You’re just yelling into the void while the real culprits collect bonuses.
Stop blaming the victim. Start burning the system down.
Bill Wolfe
December 10, 2025 AT 20:34Wow. Just wow. Someone actually wrote a thoughtful, well-researched piece about drug safety? And you all are arguing about grapefruit juice like it’s a political opinion?
Let me break this down for you, peasants: the fact that we’re still having this conversation in 2024 is a national disgrace. We have the technology to monitor every prescription in real time. We have AI that can predict interactions before they happen. We have databases with millions of reports. But we don’t have the political will to make it mandatory.
Pharmacists? They’re overworked. Doctors? They’re incentivized to prescribe, not to pause. Patients? They’re told to ‘trust the process.’
It’s not a flaw in the system. It’s the system.
And I’m not even mad. I’m just… disappointed.
🫂
Ollie Newland
December 11, 2025 AT 20:13As a pharmacist in the UK, I see this every day. We have a national system where all prescriptions are linked electronically, and we get interaction alerts pop up before we dispense. It’s not perfect, but it saves lives.
Here’s the kicker: most patients don’t know we can do that. They think we’re just the pill dispensers. We’re not. We’re the last line of defense.
And yes, we’re understaffed. And yes, we’re tired. But if you walk in with a new script and say ‘I take this and that and this tea my cousin swears by,’ we’ll stop everything to check it.
Don’t be shy. We’ve heard weirder.
Michael Feldstein
December 13, 2025 AT 08:29I love how this post doesn’t just list problems-it gives actionable steps. That’s rare.
My dad had a bad reaction to a new blood pressure med. He thought it was just ‘getting older.’ We didn’t connect it until his pharmacist noticed the timing. She called his doctor. They switched meds. He’s fine now.
It’s not about being paranoid. It’s about being informed. And you don’t need a degree to do that.
Ask. Write it down. Bring a list. Use GoodRx. Call your pharmacist. Don’t wait for a hospital visit to realize you should’ve asked sooner.
You’re not being annoying. You’re being smart.
jagdish kumar
December 14, 2025 AT 17:59Alex Piddington
December 15, 2025 AT 21:25As someone who works in health IT, I want to say: the tools are here. The data is there. The real barrier isn’t tech-it’s interoperability. Hospitals, insurers, pharmacies, and EHRs don’t talk to each other. A patient on warfarin in California might get flagged by their pharmacist, but if they travel to Texas and refill at a different chain? No one knows.
We need a national, standardized, patient-owned drug record system. Not corporate. Not government-only. Patient-controlled.
It’s possible. We’ve done it with credit scores. Why not health?
And if you’re reading this-you’re already ahead of 90% of the population. Keep going.