Bioequivalence Testing for Generic Drugs: What It Really Proves
 Dec, 15 2025
Dec, 15 2025
When you pick up a prescription and see a different name on the bottle than what your doctor wrote, it’s natural to wonder: is this generic drug really the same? You’re not alone. Millions of people in the U.S. and around the world take generic medications every day, but confusion still lingers. Some think generics are weaker, slower to work, or made with cheaper ingredients that compromise safety. The truth? Bioequivalence testing is the science that makes generics reliable - and it’s far more rigorous than most people realize.
What Bioequivalence Testing Actually Measures
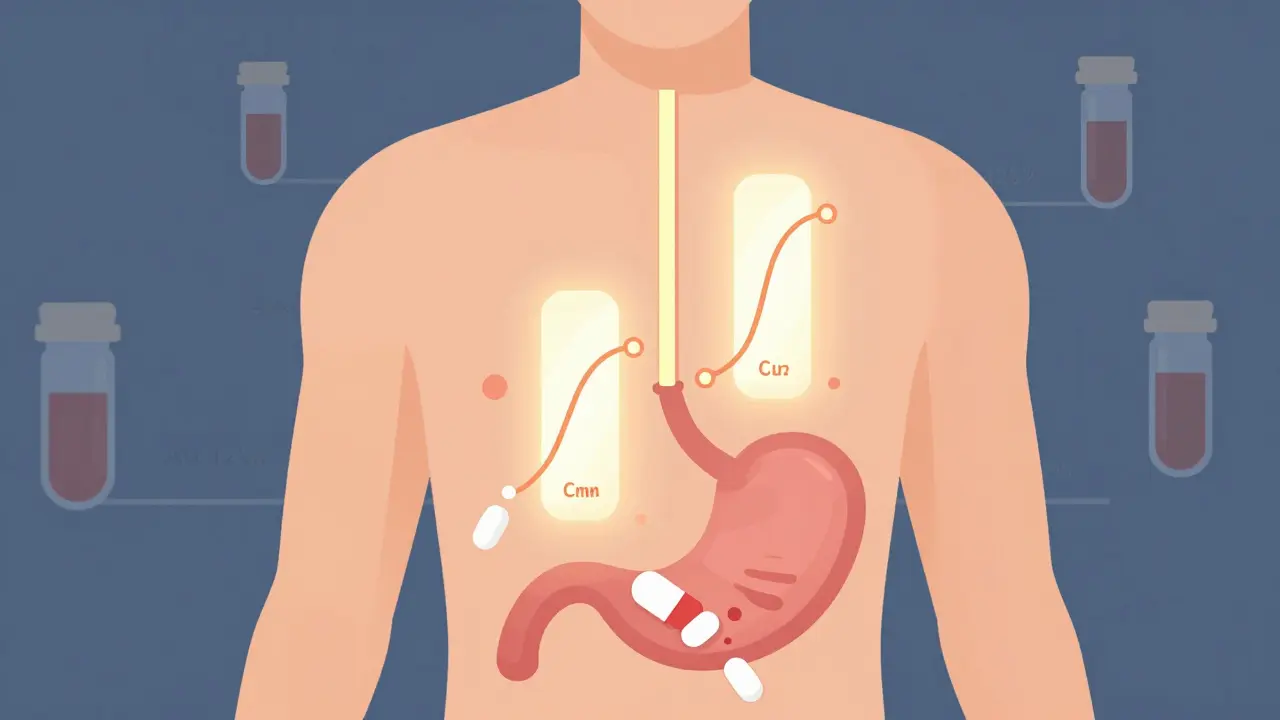
Bioequivalence testing doesn’t guess. It doesn’t rely on patient stories or anecdotal evidence. It measures exactly how your body handles the drug - from the moment you swallow it to when it’s fully absorbed into your bloodstream. The goal is simple: prove that the generic version releases the same amount of active ingredient at the same speed as the brand-name drug.
The FDA requires two key measurements: AUC (area under the curve) and Cmax (peak concentration). AUC tells you how much of the drug gets into your system overall - the total exposure. Cmax tells you how fast it gets there - the rate of absorption. For a generic to be approved, both of these values must fall within 80% to 125% of the brand-name drug’s results. That’s not a wide range. It’s a tight, scientifically validated window designed to ensure no meaningful difference in how the drug works in your body.
These tests are done in controlled studies with 24 to 36 healthy volunteers. Each person takes both the brand and the generic, in random order, under fasting conditions. Blood samples are drawn over several hours to map out exactly how the drug moves through the body. This isn’t a one-time check - the same drug is tested multiple times to ensure consistency. If even one study fails to meet the 80%-125% range, the generic doesn’t get approved.
Why This Matters More Than You Think
Many people assume that because a generic drug has the same active ingredient, it’s automatically the same. But that’s not true. Two pills with identical active ingredients can behave completely differently in your body if the coating, filler, or manufacturing process changes how quickly the drug dissolves. That’s why bioequivalence testing exists - to catch those hidden differences.
Take a common example: levothyroxine, used to treat hypothyroidism. Even small changes in absorption can affect thyroid hormone levels. That’s why the FDA has stricter bioequivalence standards for drugs like this - narrow therapeutic index drugs where tiny variations can cause real health risks. For these, the acceptable range might be tightened to 90%-111%. The system isn’t one-size-fits-all. It adapts to the drug’s risk profile.
And here’s something most people don’t know: bioequivalence testing is the reason generics cost so much less. Brand-name drugs go through years of expensive clinical trials involving thousands of patients to prove they’re safe and effective. Generics don’t repeat those trials. They use the brand’s data as a baseline - but only after proving they deliver the drug the same way. That’s the core of the Hatch-Waxman Act of 1984. It’s not a shortcut. It’s a smart, science-backed system that saves the U.S. healthcare system over $300 billion a year.
What Bioequivalence Testing Doesn’t Cover
It’s important to understand the limits. Bioequivalence testing focuses on what happens in your bloodstream. That works perfectly for pills, capsules, and liquids taken by mouth. But it doesn’t tell the whole story for drugs that act locally - like inhalers, eye drops, or topical creams.
For example, an asthma inhaler delivers medication directly to your lungs. You can’t easily measure drug levels in the lungs through blood tests. So instead of bioequivalence studies, regulators require clinical endpoint studies - proving the generic inhaler opens airways just as well as the brand. The same goes for topical corticosteroids. The FDA now requires specific in vivo studies for these products, not just dissolution tests.
Complex formulations like extended-release tablets also need extra testing. A generic version might release the drug slowly over 12 hours, but if it releases too fast at first or too slow later, it won’t work the same. That’s why some generics require multiple bioequivalence studies under different conditions - fed vs. fasted, for example - to ensure consistent performance.

Real-World Results: Do Generics Work?
Numbers don’t lie. In 2022, a Consumer Reports survey of 1,200 people found that 87% saw no difference between their generic and brand-name drugs. Nine percent said the generic worked better. Only 4% reported it worked less effectively. Most of those who noticed a difference pointed to side effects like stomach upset or headaches - not because the drug didn’t work, but because of inactive ingredients like dyes or fillers.
On Reddit’s r/pharmacy community, a 2023 thread with over 1,400 comments showed that 78% of users reported no difference at all. Those who did notice changes often switched back and forth between brands and generics and found the effects identical - except for pill color or size.
Still, myths persist. A 2021 study found that 32% of patients believed generics were less effective. That’s not because the science is flawed. It’s because the messaging hasn’t caught up. Pharmacies, doctors, and manufacturers need to do better at explaining what bioequivalence really means.
How the System Keeps Generics Safe
Approval isn’t the end. The FDA inspects around 1,200 generic drug manufacturing sites every year - both in the U.S. and overseas. These aren’t random checks. They’re full audits of production lines, quality control labs, and documentation systems. If a facility fails, the FDA can block shipments or pull approval.
Every generic drug must meet the same strict standards for identity, strength, purity, and stability as the brand. The only legal differences allowed are in color, shape, flavor, and inactive ingredients. That’s why your generic might look different - but the active ingredient? Identical. And the way your body processes it? Proven to be the same.
The FDA’s Orange Book lists every approved generic and its therapeutic equivalence rating. If a generic is rated AB, it means the FDA has determined it’s therapeutically equivalent to the brand. You can trust that rating. It’s not marketing. It’s science.

What’s Changing in Bioequivalence Testing
The field is evolving. The FDA is moving toward using computer modeling to predict how a drug will behave in the body - a method called physiologically based pharmacokinetic (PBPK) modeling. This isn’t replacing human studies yet, but it’s helping reduce the number of volunteers needed for certain complex drugs.
For example, instead of testing 30 people for a new inhaler, a model might predict performance based on lung anatomy and drug properties. Then, only a few human tests are needed to confirm. This makes development faster and cheaper - without lowering standards.
The European Medicines Agency and other global regulators are following the same path. Harmonized guidelines from the ICH mean that a generic approved in the U.S. is likely to meet standards in Europe, Japan, and Canada. That’s why you can trust generics from different countries - the science behind them is aligned.
Bottom Line: You Can Trust Bioequivalence
Generic drugs aren’t a compromise. They’re the result of decades of refined science, strict regulation, and global cooperation. Bioequivalence testing is the gatekeeper that ensures every generic you take performs just like the brand. It’s not perfect - no system is - but for over 90% of prescriptions, it works exactly as intended.
If you’ve had a bad experience with a generic, it’s likely due to inactive ingredients - not the active drug. Talk to your pharmacist. They can help you switch to a different generic version or check if your medication falls into a category that needs special testing, like thyroid meds or seizure drugs.
The bottom line? When your doctor prescribes a generic, you’re not getting a second-rate drug. You’re getting the same medicine, tested the same way, approved under the same rules - for a fraction of the cost. And that’s not just smart healthcare. It’s science working the way it should.
Are generic drugs as effective as brand-name drugs?
Yes, when approved by the FDA, generic drugs are required to be therapeutically equivalent to their brand-name counterparts. Bioequivalence testing proves they deliver the same amount of active ingredient at the same rate, ensuring identical effectiveness in the body. Over 90% of prescriptions in the U.S. are filled with generics, and studies show most patients experience no difference in outcomes.
How long does bioequivalence testing take?
A typical bioequivalence study with healthy volunteers takes 6 to 8 months to complete, including study design, recruitment, dosing, blood sampling, and data analysis. This is part of the overall ANDA approval process, which usually takes 10 to 12 months from submission to final approval. Complex drugs may require additional studies, extending the timeline.
Why do generic pills look different from brand-name pills?
By law, generic drugs must look different from brand-name drugs to avoid trademark infringement. This means differences in color, shape, size, or flavor are allowed - but only in inactive ingredients. The active ingredient, strength, dosage form, and bioavailability must be identical. The appearance has no effect on how the drug works.
Can I trust generics made overseas?
Yes. The FDA inspects all manufacturing facilities - whether in the U.S., India, China, or elsewhere - using the same standards. Over 1,200 generic drug plants are inspected annually. A generic drug approved by the FDA meets the same quality, safety, and bioequivalence requirements regardless of where it’s made. The origin doesn’t determine quality - the regulatory process does.
Do bioequivalence tests apply to all types of medications?
No. Bioequivalence testing using blood samples works best for oral medications that enter the bloodstream. For inhalers, eye drops, topical creams, or injectables, different methods are used - such as clinical endpoint studies or pharmacodynamic tests - because the drug acts locally and can’t be measured accurately through blood levels. The FDA has specific guidelines for each type of product to ensure equivalence.
What are narrow therapeutic index drugs, and why are they special?
Narrow therapeutic index (NTI) drugs have a small margin between an effective dose and a toxic one. Examples include warfarin, levothyroxine, and some seizure medications. For these, the FDA applies stricter bioequivalence standards - often tightening the acceptable range from 80%-125% to 90%-111%. This ensures even tiny differences in absorption don’t lead to dangerous side effects or loss of effectiveness.

anthony epps
December 16, 2025 AT 10:36I always thought generics were just cheap copies, but this actually makes sense. The 80-125% range isn't wide at all if you think about it. My blood pressure med switched last year and I didn't notice a thing.
Dan Padgett
December 17, 2025 AT 16:04Man, this reminds me of how we used to think the moon was made of cheese back in the village. Science ain't magic, but it sure feels like it when it works this smooth. Generics? They ain't second-rate-they're second-born, same soul, different wrapper.
Hadi Santoso
December 18, 2025 AT 14:55So i just learned that the FDA inspects like 1200 plants a year?? that's wild. i always assumed the cheap pills were made in some basement in bangladesh with no oversight. turns out they're probably held to the same standards as my local CVS. also, the orange book?? that's such a chill name for something so serious. like a drug version of the yellow pages but way more life-saving.
Kim Hines
December 19, 2025 AT 17:37My mom switched to generic levothyroxine and her TSH went haywire. She had to go back to brand. Not because it didn't work-but because the fillers upset her stomach. So yeah, the science is solid, but bodies aren't all the same.
Aditya Kumar
December 21, 2025 AT 07:22whatever
Randolph Rickman
December 21, 2025 AT 16:25Let me break this down real simple: if you're scared of generics, you're scared of math. The FDA doesn't play. They test it, they check it, they double-check it. And if it passes? It's the same drug. No magic. No trick. Just science saving you hundreds a year. Stop letting fear drive your pharmacy choices.
sue spark
December 23, 2025 AT 04:19I never realized how much goes into this. I thought it was just the same pill with a different color. Turns out it's a whole system of blood tests and timing and fasting. Makes me feel better about switching. Also why do they call it the orange book? That's so random
Tiffany Machelski
December 23, 2025 AT 08:56My cousin switched to generic adderall and said it made her feel 'off'-but it was the dye. She's allergic to red 40. So yeah, it's not the drug, it's the junk they put around it. Always ask your pharmacist about fillers.
SHAMSHEER SHAIKH
December 24, 2025 AT 02:46Dear esteemed colleagues and conscientious citizens of the healthcare ecosystem, I must express my profound admiration for the rigorous, meticulously calibrated, and globally harmonized framework of bioequivalence testing as elucidated herein. The scientific fidelity, regulatory discipline, and economic wisdom embodied in the Hatch-Waxman Act constitute nothing short of a triumph of rational governance over commercial opportunism. May we never underestimate the dignity of the generic pill-unadorned, unbranded, yet unyieldingly potent in its therapeutic fidelity.
James Rayner
December 24, 2025 AT 14:08It's wild how much we trust pills without thinking about them. Like, I just swallow them and move on. But this? This is like watching a magician reveal how the trick works-and it's not magic, it's just really good math. 🤯
Andrew Sychev
December 26, 2025 AT 11:18Oh great, another article telling me I'm dumb for doubting generics. Newsflash: I had a seizure after switching. Maybe your 'science' doesn't work for everyone. And no, I'm not crazy. I'm just the one who got screwed because someone thought 80-125% was 'close enough'.
Arun ana
December 27, 2025 AT 22:37So if generics are so good, why do some doctors still write 'do not substitute'? I think it's because they're used to the brand. But honestly, I've been on generic metformin for 5 years and I'm fine. No issues. Just a cheaper pill.
Kayleigh Campbell
December 28, 2025 AT 18:26So the FDA spends millions testing pills so we can save $300 billion a year... and yet somehow my pharmacy still charges $40 for a 30-day supply of generic insulin. Someone's making a profit. Just not me.
Dave Alponvyr
December 30, 2025 AT 16:03Generics work. If yours didn't, it's the filler, not the drug. Talk to your pharmacist. Done.